Phase State Diagram For Gasoline. Point a represents the single dense phase fluid outside the phase envelope. The properties of the microscopic state change by definition at the phase boundary.
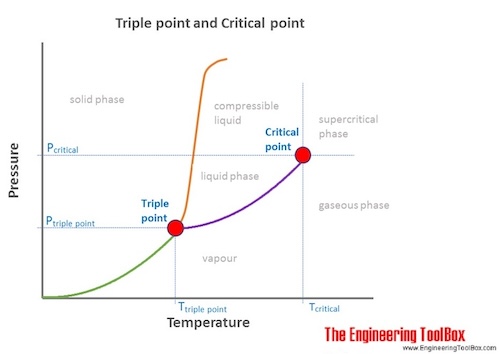
The markings show points where two or more phases can co exist in equilibrium. The essential level is the tip level of a phase equilibrium curve outlined by a essential stress tp and demanding temperature computer. These binary systems with.
These binary systems with.
Thus much of the phase diagram at low temperatures is dominated by a 2 phase field of two different solid structures one that is highly enriched in component a the α phase and one that is highly enriched in component b the β phase. As pressure is lowered point b is reached where condensation begins. These data are largely uncertain or unknown 10 and so are omitted from the diagram the appendix lists the equations whether fitted or theoretical for carbon dioxide equilibria or the experimental data used figure 1. Line abde represents a typical isothermal constant temperature retrograde condensation process.