Fcc Unit Cell Diagram. Now similarly each face center contributes to two unit cells so contribution per unit cell by six face centers is equal to 21. Note that although the unit cell in these crystals is conventionally taken to be a cube the primitive unit cell often is not.
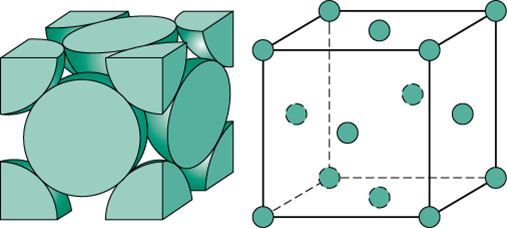
Interactive model of this structure type at chemtube3d. 1 fcc unit cell has 4 atoms while 1 bcc unit cell has 2 atoms volume change v fcc 2v bcc 2 vbcc 0046307 2 0023467 x100 2 0023467 134 iron contracts upon heating. They are called simple cubic face centred cubic and body centred cubic.
Ld 110 2 atom4r 12r ld 001 1 atom2r2 12r 2 ld 111 1 atoms4r 12r 6.
Th part of 8 corner atoms ie 1 atom. Fcc structure in this structure the unit cell contains atoms at the centre of the faces of the unit cell and thus it leads to naming it as face centred. They vary in how the atomsspheres are arranged inside of it. In addition there are 6 atoms at the face centers of the cube.