Energy Level Diagram Hydrogen Atom Bohr Model Figure 4 5. Figure 519 bohr model for hydrogen. According to bohrs theory electrons of an atom revolve around the nucleus on certain orbits or electron shells.
His postulates explaining the energy level are discussed below. Bohr proposed an atomic model of a hydrogen atom. If an electron gains energy it can move to a higher energy level.
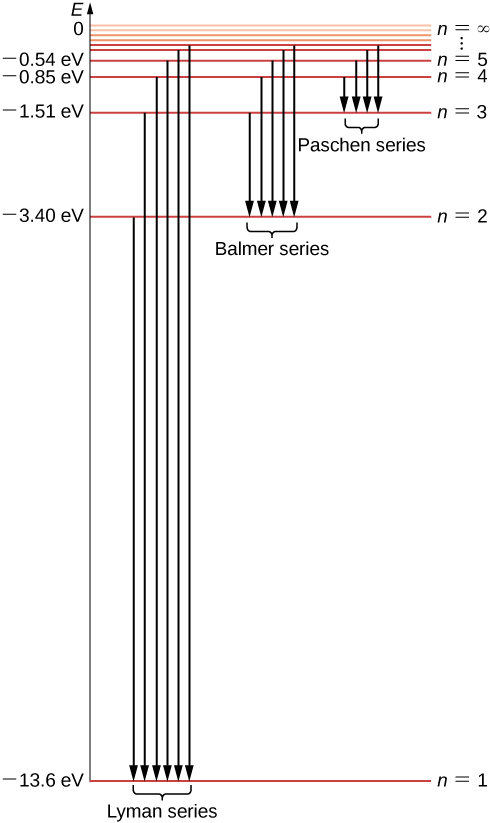
The hydrogen atom has the simplest energy level diagram.
Seven electrons are trapped in a one dimensional infinite potential well of width lwhat multiple of 8 m l 2 h 2 gives the energy of a the first excited state b the second excited state and c the third excited state of the system of seven elec trons. Bohrs model explained how electrons travel in different circular orbits around the nucleus. 5 arises from the transition from level b to a. Energy level diagram.